| Kidney Res Clin Pract > Volume 32(2); 2013 > Article |
|
Abstract
Pseudomonas stutzeri is a Gram-negative, rod-shaped, motile, single polar-flagellated, soil bacterium that was first isolated from human spinal fluid and is widely distributed in the environment. It was isolated as an uncommon opportunistic pathogen from humans, and a few cases of P. stutzeri-induced peritonitis have been reported in patients undergoing continuous ambulatory peritoneal dialysis (CAPD). Catheter removal with antibiotic treatment is generally recommended because peritonitis by Pseudomonas species is commonly associated with catheter-related infection. Here, we describe the first case of P. stutzeri-induced peritonitis in an 82-year-old woman in Korea. She had received two antipseudomonal antibiotics, an aminoglycoside (isepamicin, Yuhan corporation, Seoul, Korea) and a fluoroquinolone (ciprofloxacin), and was successfully treated without removal of the CAPD catheter.
Keywords
Antibiotic treatment, Peritoneal dialysis, Peritonitis, Pseudomonas stutzeriThe incidence of peritonitis in patients undergoing continuous ambulatory peritoneal dialysis (CAPD) has significantly decreased recently compared to rates in the past because of the development of peritoneal dialysis methods and catheter-related techniques, continuous patient education, and the establishment of antibiotic treatment guidelines; however, peritonitis remains an important cause of peritoneal dialysis failure [1]. In peritonitis induced by a single microorganism strain, coagulase-negative staphylococcus was the most common cause of peritonitis (24.3%), followed by Streptococcus, Staphylococcus, Escherichia coli, and Pseudomonas aeruginosa. Peritonitis induced by multiple microorganism strains has been reported in 7% of cases [2].
Pseudomonas stutzeri was first isolated from human spinal fluid as a Gram-negative motile organism with a single polar flagella. P. stutzeri is widely distributed in the environment and rarely causes infections, but it has been isolated as an opportunistic pathogen in clinical conditions [3].
P. stutzeri-induced peritonitis in patients with CAPD was reported by Ceri et al. [4] overseas, but no cases have so far been reported in Korea. With regard to inflammatory diseases other than peritonitis, only one case of choroid plexitis has been reported domestically [5]. In this report, we present a case of peritonitis by an uncommon pathogen, P. stutzeri, in a patient undergoing CAPD treated without catheter removal.
An 82-year-old woman who had undergone CAPD for 1 year for the treatment of end-stage renal disease secondary to hypertension was hospitalized for a cloudy peritoneal dialysis effluent for 1 day before admission. She had a history of cholecystectomy due to gallstones at 72 years of age.
Physical examination on admission revealed abdominal distension, decreased bowel sounds, and mild diffuse abdominal tenderness. The peritoneal catheter site displayed no erythema or discharge. The patient’s vital signs were as follows: body temperature, 36.8 °C; blood pressure, 185/88 mmHg; and heart rate, 78/min in sinus rhythm.
Laboratory analysis revealed the following: white blood cell count of 10.4×103/mm3 with 89% polymorphonucleocytes; hemoglobin, 11.1 g/dL; platelet count, 304×103/mm3; erythrocyte sedimentation rate, 64 mm/h; C-reactive protein level, 2.92 mg/dL. The patient’s blood chemistries were normal except for a creatinine level of 6.33 mg/dL, blood urea nitrogen level of 56.7 mg/dL, and albumin level of 2.8 g/dL. Chest radiography showed mild pulmonary congestion with cardiomegaly, whereas abdominal radiography revealed a mild paralytic ileus (Fig. 1). Peritoneal fluid analysis revealed a leukocyte count of 9.92×103/mm3 (polymorphonuclear leukocytes, 84%; lymphocytes, 4%).
Empirical therapy was initiated with intraperitoneal cefazolin (Dong-A pharmaceutical company limited, Seoul, Korea) and isepamicin. Because P.stutzeri grew in the culture of the peritoneal effluent performed on admission, cefazolin was discontinued from the 9th day of peritonitis, and ciprofloxacin was administered orally in addition to the initial treatment. The total treatment period was 3 weeks. The white blood cell count of the peritoneal fluid decreased from 9,920/mm3 on admission to 6/mm3 on the 19th day of peritonitis, and the dialysate effluent that was cloudy on admission drained clear from the 3rd day of peritonitis. There were no other complications, and the patient is currently undergoing CAPD through the outpatient nephrology service without recurrent peritonitis.
Identifying the causative microorganism is important for antibiotic selection and the determination of a treatment strategy in the management of CAPD peritonitis. Clinical features and treatment methods vary according to the causative microorganism. Approximately 72% of CAPD peritonitis cases can be treated with antibiotics, but the catheter must be removed in the other 28% of cases because of recurrent and intractable peritonitis such as fungal infections [6].
P. stutzeri is a widely distributed Gram-negative bacterium that rarely causes infections. However, it has been isolated as an opportunistic pathogen in immunocompromised hosts. Cases of P. stutzeri have been reported in the form of osteomyelitis, arthritis, endocarditis, meningitis, pneumonia, empyema, skin infections, eye infections, urinary tract infections, and diverticulitis, and susceptibility tests for several antibiotics have been performed in epidemiological and case reports.
Nearly all studies involving several antibiotics and bacterial species have shown that P. stutzeri is sensitive to many more antibiotics than P. aeruginosa, its most closely related species and a well-known human pathogen [3]. The higher sensitivity of P. stutzeri can be explained by its reduced exposure to antibiotics because of its low incidence rate in clinical environments. Despite these results, when isolated in immunocompromised hosts, P. aeruginosa and other Pseudomonas spp., including P. stutzeri, did not show any significant difference [7]. This finding implies that P. stutzeri has a range of antibiotic resistance mechanisms, such as changes in outer membrane proteins and lipopolysaccharide, and the presence of β-lactamase has been described [8].
Siva et al. [9] reported that Pseudomonas is associated with a higher catheter removal rate and conversion to hemodialysis compared to general microorganisms, and immediate removal of the peritoneal catheter and treatment with two types of antipseudomonal antibiotics decreased mortality through a meta-analysis of 191 cases of peritonitis by Pseudomonas that occurred in 2003–2006 in Australia. P. stutzeri was identified in 15 (8%) of a total of 191 cases.
Ceri et al. [4] treated the patients with refractory CAPD peritonitis due to P. stutzeri with susceptible antibiotics and catheter removal. Tan et al. [10] described P. stutzeri as one of the causative pathogens of recurrent peritonitis in pediatric patients undergoing CAPD. However, no case has yet been reported in Korea.
In this case, the patient was treated with antibiotics without catheter removal because the isolated bacterium was susceptible to ciproflocaxin and isepamicin (Table 1), there was no evidence of an exit site infection or tunnel infection, and her clinical symptoms improved after antibiotic administration (Table 2).
According to the recommendations of the International Society for Peritoneal Dialysis, catheter removal with antibiotic treatment is recommended because peritonitis by Pseudomonas species, which has the capacity to generate biofilm, is commonly associated with catheter-related infection [9], [11]. In addition, like P. aeruginosa, P. stutzeri has a variety of antibiotic resistance mechanisms. Thus, the administration of two susceptible antibiotics with different mechanisms of action is required [12].
In conclusion, management of P. stutzeri-induced peritonitis should be the same as that with P. aeruginosa. However, careful deliberation is necessary upon CAPD catheter removal because P. stutzeri infection progresses according to host immunity and antibiotic exposure as shown in this case.
References
1. Perit Dial Int.
2. Han SH, Lee SC, Ahn SV, Lee JE, Choi HY, Kim BS, Kang SW, Choi KH, Han DS, Lee Hy. Improving outcome of CAPD: twenty-five years’ experience in a single Korean center. Perit Dial Int 27:2007;432–440.


3. Noble RC, Overman SB.. Overman Pseudomonas stutzeri infection — a review of hospital isolates and a review of the literature. Diagn Microbiol Infect Dis 19:1994;51–56.


4. Ceri M, Ortabozkoyun L, Altay M, Unverdi S, Kurultak I, Huddam B, Kilik F, Yilmaz R, Duranay M. Peritonitis due to Pseudomonas stutzeri, an organism that may be difficult to culture. Perit Dial Int 30:2010;484–486.


5. Kim NG, Kwon OY, Park KJ, Choi NC, Lim BH. A case of entrapped temporal horn of lateral ventricle caused by Pseudomonas stutzeri choroid plexitis. J Korean Neurol Assoc 15:1997;421–428.
6. Kim GC, Korbet SM. Polymicrobial peritonitis in continuous ambulatory peritoneal dialysis patients. Am J Kidney Dis 36:2000;1000–1008.


7. Tattawasart U, Maillard JY, Furr JR, Russell AD. Comparative responses of Pseudomonas stutzeri and Pseudomonas aeruginosa to antibacterial agents. J Appl Microbiol 87:1999;323–331.


8. Tattawasart U, Maillard JY, Furr JR, Russel AD. Development of resistance to chlorhexidine diacetate and cetylpyridinium chloride in Pseudomonas stutzeri and changes in antibiotic susceptibility. J Hosp Infect 42:1999;219–229.


9. Siva B, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins K.J., Bannister KM, Johnson DW, McDonald SP. Pseudomonas peritonitis in Australia: predictors, treatment, and outcomes in 191 cases. Clin J Am Soc Nephrol 4:2009;957–964.



10. Tan R, Betjes M, Cransberg K. Post-transplantation encapsulating peritoneal sclerosis in a young child. Nephrol Dial Transplant 26:2011;3822–3824.


Figure 1
Abdominal radiograph. Paralytic ileus and the correct position of a continuous ambulatory peritoneal dialysis(CAPD) catheter with its tip with in the pelvis are shown.
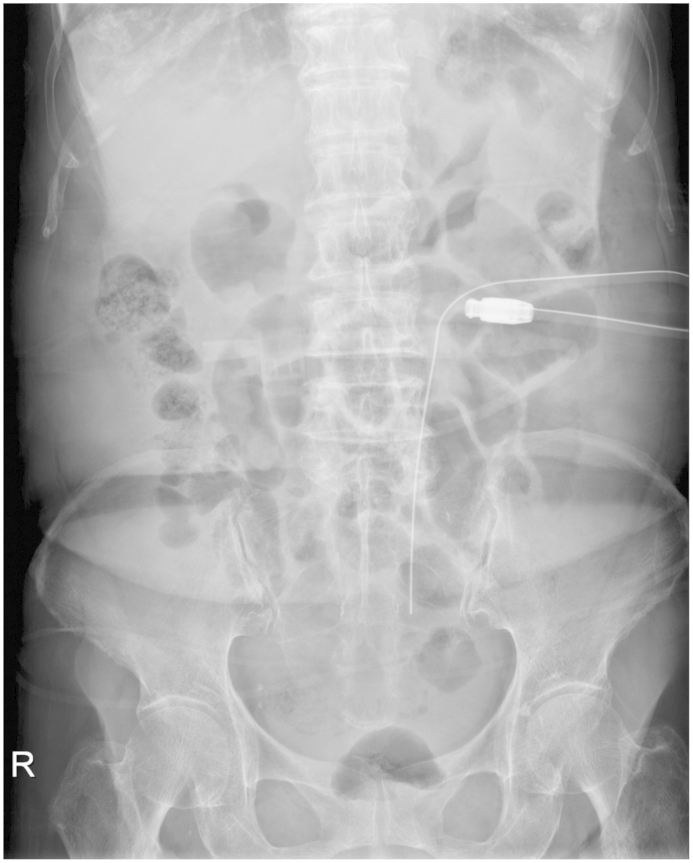
Table 1
Antibiotic susceptibility testing results from the patient’s peritoneal fluid



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















